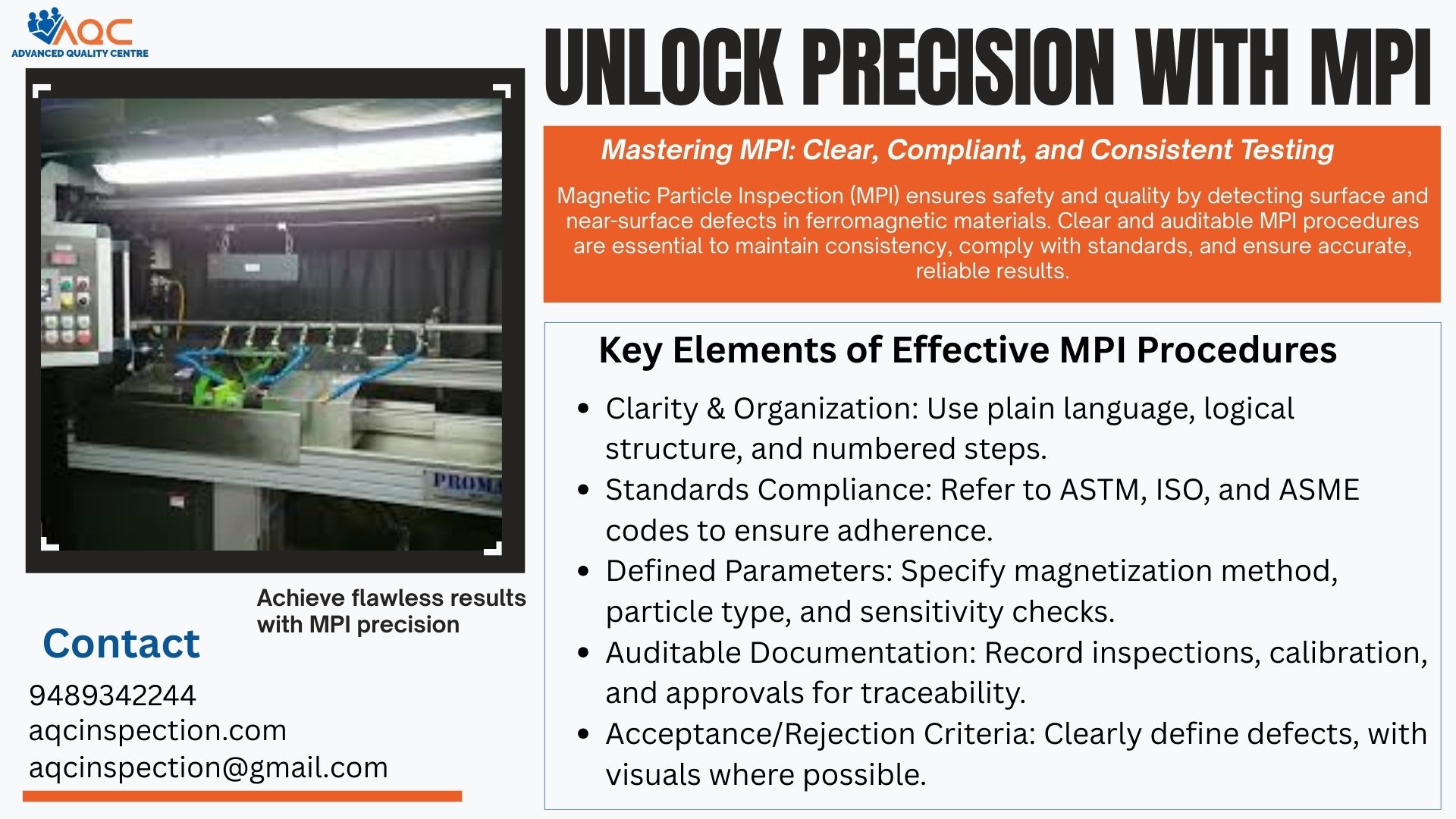
Quality Assurance Glossary
This blog gives you the abbreviations and definitions of terms used in quality Assurance department.
Abbreviations
The following abbreviations apply to this Project Quality Plan:
- BOM Bill of Material
- CAV Characteristic Accountability and Verification
- CAR Corrective Action Request
- CAPA Corrective Action & Preventive Action
- CTQ Critical to Quality
- CTP Critical to Process
- CpK Process Capability Index
- EMPIS Engineering Materials & Process Information Services
- FMEA Failure Modes & Effects Analysis
- FPQ First Piece Qualification
- GE General Electric
- HPI High Precision Industry
- ITP Inspection Test Plan
- ISO 9001 Quality Management System Requirement Standard
- MPP Manufacturing Process Plan
- NDT Non Destructive Testing
- NCR Non-Conformance and Corrective Action Report
- PO Purchase Order
- PQP Project Quality Plan
- PQR Procedure Qualification Record
- QA Quality Assurance
- QAP Quality Assurance Procedure
- QC Quality Control
- QHSE Quality, Health, Safety and Environment
- RCA Root Cause Analysis
- SDR Supplier Deviation Request
- RFQ Request for Quotation
- TPI Third Party Inspection Agency
- WO Work Order
- WI Works Instruction
- SOW Scope of Work
- WPS Welding Procedure Specification
Definitions
Characteristic Accountability and Verification (CAV) – is a document applied to all production components which ensures controlled processes for maintaining drawing features and characteristics.
CAV shall be included a minimum of the following components
- Identification of components
- Characteristics and feature accountability
- Inspection and test results
- Production product acceptance criteria
Failure Modes & Effects Analysis (FMEA) is one example of an accepted process risk assessment format, where the supplier will perform a risk assessment of its manufacturing and quality assurance processes to evaluate the effectiveness of these processes to consistently produce the component or provide the qualified service.
Bill of Material (BOM) is defined as the list of materials down to its detailed component level. At the time of the supplier design review during the qualification and prior to entering production.
Purchase Order (P.O) – is the document which gives the requirement of the company, such as parts , drawings and services, and the requirement shall not be deviated by the supplier with out an company’s approved deviation request.
First Piece Qualification (FPQ) and Pilot Lot Qualification (PLQ). FPQ requires the supplier to manufacture a first piece of the item using the same process, people, parts, and systems as the planned production environment.
Containment: Containment actions can be focused on the product in which the nonconformance was detected as well as focused on similar products or product families in which the nonconformance may occur.
Correction: Action to eliminate a detected nonconformance, defect or other undesirable situation.
Corrective Action: Action taken to eliminate the cause(s) of an existing nonconformance, defect or other undesirable situation to prevent recurrence.
Corrective Action Request: Is a document format to express the non conformance to project quality needs had occurred, and requires root cause analysis and corrective action.
CpK study/data: report process capability and process performance through statistical measurements
Critical to Quality (CTQ) Characteristics: Internal critical to quality parameters that relate to the wants and needs of the customer. Also called critical to process (CTP) characteristics.
Gage R&R: Gage repeatability and reproducibility, is a statistical tool that measures the amount of variation in the measurement system arising from the measurement device and the people taking the measurement.
Frozen Process: A manufacturing method/process/procedure/control that is approved by the client and cannot be deviated.
Manufacturing Process Plan (MPP): A detailed, step-by-step list of operations and requirements by which components or services are manufactured.
Non-Destructive Testing (NDT): Analysis techniques used to evaluate properties of material, component or system without causing damage. Typical methods would include ultrasonic, magnetic-particle, liquid penetrant, radiography, eddy current testing, etc.
Preventive Action: Action taken to eliminate the cause(s) of a potential nonconformance or undesirable potential situation to prevent occurrence.
Product Quality Plan (PQP): A detailed, step-by-step list of operations and requirements in which a supplier identifies a process of how, what, why, when and who will perform tests or inspections and the applicable acceptance criteria. This may also be referred to as an Inspection and Test Plan (ITP).
Qualification Requirements: All required documentation for qualification of manufacturer
Repair: A type of correction performed to a nonconformance that reduces but not completely eliminates the nonconformance(s) such that the product is determined to be usable for its intended purpose.
Request for Design Change: A document submitted by the supplier to request the customer a change of design in their drawing
Rework: A type of correction performed to a nonconformance that completely eliminates the nonconformance(s) such that the product conforms to the specification or requirement.
Scrap: A disposition for nonconforming product that is not useable for its intended purpose and that cannot be economically reworked or repaired in an acceptable manner.
Special Process: A process by which results cannot be fully verified through subsequent nondestructive inspection and testing of the product and where processing deficiencies may become apparent only after the product is in use.
Additionally, processes that require operators of that process to be qualified and certified to be able to conduct the process and meet technical regulations and standards are considered special processes.
Supplier Deviation Request (SDR): A request initiated by the supplier to deviate from purchase order technical requirements (drawings, specifications, engineering instructions, etc.) or the approved qualification package.
Process Capability Index (CpK): is a measurement tool which can be used to calculate a baseline value for monitoring the improvement or lack of improvement in the process, the tool can be used for measurement of results after making improvement in the project.
Example:
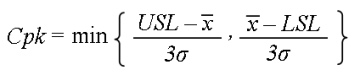
Let’s assume the following information:
Lower Specification Limit (LSL): 5mm
Upper Specification Limit (USL): 10mm
The mean of your data set consisting of 50 observations is normal and the value is 7.85mm. The standard deviation is 0.23mm
The mean of your data set consisting of 50 observations is normal and the value is 7.85mm. The standard deviation is 0.23mm
Find Cpk:
Find both values by substituting the values into the formula at the top of this page
1: (10mm – 7.85mm) / 3*0.63mm = 2.15mm / 1.89mm = 1.14
- (7.85mm – 5mm) / 3*0.63mm = 2.85mm / 1.89mm = 1.51
The minimum value is used; therefore, the Cpk = 1.14
Inspection – The examination, measurement and testing of the characteristics of products and/or services to determine acceptability and record inspection and test data.
Non-Conformance – A deficiency in characteristics, documentation or procedure, which renders the quality of a product or service unacceptable or indeterminate or not according to specified requirements. Examples of non-conformance are the following: physical defects, test failures, inadequate documentation, and deviations from prescribed processing or from any other part of the program.
Procedure – A document that specifies, as applicable, the purpose and scope of an activity; what shall be done and by whom; when and how it shall be done; what materials, equipment and documentation shall be used and how it shall be controlled.
Production – All activities involved in the fabrication, assembly and testing of products to specified requirements.
Progress Report – A document that gives details of the work planned and what actually achieved in discipline wise, on timely basis .
Quality – The features and characteristics of products or services that bear on their ability to meet specified requirements.
Quality Control – Part of quality management focused on fulfilling quality requirements.
Quality Assurance – All those planned and systematic actions needed to provide adequate confidence that products and/or services will satisfy specified requirements.
Quality Assurance Procedure – An document which details the systematic approach in align the suppliers quality management system and purchases quality requirements along with the requirements of regulatory authority
Quality Audit – A documented activity aimed at verifying, by independent examination and evaluation, that the applicable elements of the quality assurance program have been established, documented and implemented effectively in accordance with specified requirements.
Regulatory Authority – The Federal or Municipal agency having the lawful right and power to interpret the law and exercise authority.
Supplier – vendor/Service provider/Sub-Subcontractor of the materials and equipment to the SUB CONTRACTOR
Subcontract – A contract between CONTRACTOR and SUB CONTRACT
SUB CONTRACTOR – The party responsible for the performance of the work.
To learn more about the Quality Assurance glossary ,quality management tools, Quality training on mechanical testing, Quality controm for mechanical Engineers contact us at https://aqcinspection.com/training/
Visit our technical and career updates at our Blog site https://advancedqualitycentre.blogspot.com . or https://ndtcenter.blogspot.com